THUNDER样机最新应用展示 | 天津医科大学赵丽课题组
刊登杂志:
naturecommunications
影响因子:
16.6
文章题目:
Proteostatic reactivation of the developmental transcription factor TBX3 drives BRAF/MAPK-mediated tumorigenesis
用户机构:
天津医科大学赵丽课题组

摘要:
MAPK pathway-driven tumorigenesis, often induced by BRAFV600E, relies on epithelial dedifferentiation. However, how lineage differentiation events are reprogrammed remains unexplored. Here, we demonstrate that proteostatic reactivation of developmental factor, TBX3, accounts for BRAF/MAPK-mediated dedifferentiation and tumorigenesis. During embryonic development, BRAF/MAPK upregulates USP15 to stabilize TBX3, which orchestrates organogenesis by restraining differentiation. The USP15-TBX3 axis is reactivated during tumorigenesis, and Usp15 knockout prohibits BRAFV600E-driven tumor development in a Tbx3-dependent manner. Deleting Tbx3 or Usp15 leads to tumor redifferentiation, which parallels their overdifferentiation tendency during development, exemplified by disrupted thyroid folliculogenesis and elevated differentiation factors such as Tpo, Nis, Tg. The clinical relevance is highlighted in that both USP15 and TBX3 highly correlates with BRAFV600E signature and poor tumor prognosis. Thus, USP15 stabilized TBX3 represents a critical proteostatic mechanism downstream of BRAF/MAPK-directed developmental homeostasis and pathological transformation, supporting that tumorigenesis largely relies on epithelial dedifferentiation achieved via embryonic regulatory program reinitiation.
本研究表明,发育因子TBX3的蛋白抑制再激活,解释了BRAF/ mapk介导的去分化和肿瘤发生。在胚胎发育过程中,BRAF/MAPK上调USP15以稳定TBX3, TBX3通过抑制分化来协调器官发生。Usp15 - tbx3轴在肿瘤发生过程中被重新激活,Usp15敲除以tbx3依赖的方式阻止BRAFV600E驱动的肿瘤发展。删除Tbx3或Usp15会导致肿瘤再分化,这与它们在发育过程中的过度分化倾向相似,例如甲状腺滤泡发生中断和分化因子如Tpo、Nis、Tg升高。研究结果表明,USP15和TBX3均与BRAFV600E特征和肿瘤预后不良高度相关。因此,USP15稳定的TBX3代表了BRAF/ mapk导向的发育稳态和病理转化下游的关键蛋白抑制机制,支持肿瘤发生主要依赖于通过胚胎调控程序重新启动实现的上皮去分化。
THUNDER 应用
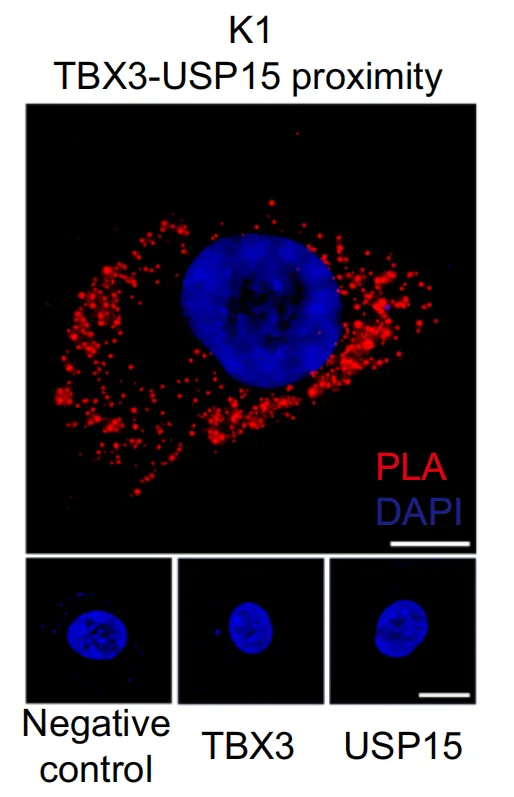
采用PLA法验证USP15和TBX3在K1细胞中的共定位。